Organic chemistry

Methane, CH4; the line-angle structural formula shows four carbon-hydrogen single bonds (σ, in black), and the typical 3D shape of tetrahedral molecules, with ~109° interior bond angles (in dashed-green).
Organic chemistry is a chemistry subdiscipline involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.[1]Study of structure includes many physical and chemical methods to determine the chemical composition and the chemical constitution of organic compounds and materials. Study of properties includes both physical properties and chemical properties, and uses similar methods as well as methods to evaluate chemical reactivity, with the aim to understand the behavior of the organic matter in its pure form (when possible), but also in solutions, mixtures, and fabricated forms. The study of organic reactions includes probing their scope through use in preparation of target compounds (e.g., natural products, drugs, polymers, etc.) by chemical synthesis, as well as the focused study of the reactivities of individual organic molecules, both in the laboratory and via theoretical (in silico) study.
The range of chemicals studied in organic chemistry include hydrocarbons (compounds containing only carbon and hydrogen), as well as myriad compositions based always on carbon, but also containing other elements,[1][2][3]especially oxygen, nitrogen, sulfur, phosphorus (these included in many organic chemicals in biology) and the radiostable elements of the halogens.
In the modern era, the range extends further into the periodic table, with main group elements, including:
- Group 1 and 2 organometallic compounds, i.e., involving alkali (e.g., lithium, sodium, and potassium) or alkaline earth metals (e.g., magnesium)
- Metalloids (e.g., boron and silicon) or other metals (e.g., aluminium and tin)
In addition, much modern research focuses on organic chemistry involving further organometallics, including the lanthanides, but especially the transition metals; (e.g., zinc, copper, palladium, nickel, cobalt, titanium and chromium)
Three representations of an organic compound, 5α-Dihydroprogesterone (5α-DHP), a steroid hormone. For molecules showing color, the carbon atoms are in black, hydrogens in gray, and oxygens in red. In the line angle representation, carbon atoms are implied at every terminus of a line and vertex of multiple lines, and hydrogen atoms are implied to fill the remaining needed valences (up to 4).
Finally, organic compounds form the basis of all earthly life and constitute a significant part of human endeavors in chemistry. The bonding patterns open to carbon, with its valence of four—formal single, double, and triple bonds, as well as various structures with delocalized electrons—make the array of organic compounds structurally diverse, and their range of applications enormous. They either form the basis of, or are important constituents of, many commercial products including pharmaceuticals; petrochemicals and products made from them (including lubricants, solvents, etc.); plastics; fuels and explosives; etc. As indicated, the study of organic chemistry overlaps with organometallic chemistry and biochemistry, but also with medicinal chemistry, polymer chemistry, as well as many aspects of materials science.[1]
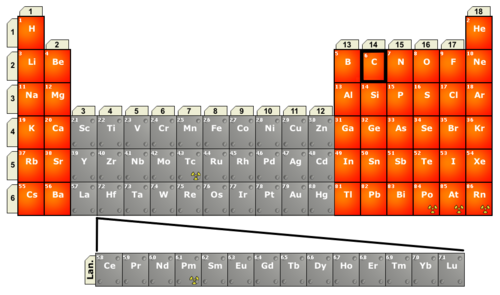
Periodic table of elements of interest in organic chemistry. The table illustrates all elements of current interest in modern organic and organometallic chemistry, indicating main group elements in orange, and transition metals and lanthanides (Lan) in grey.


Comments
Post a Comment